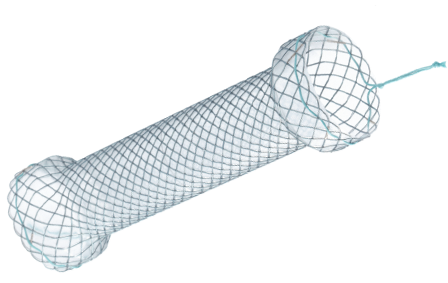
Esophageal S Type stent
| Category: Full and partially covered |
Indication
Benign and malignant esophageal strictures, esophageal fistulas / leaks
Details / Features
- Fixed cell with braided construction
- High flexibility and optimal radial force
- Both head ends (8 mm larger than trunk) help minimize migration
- Silicone covering and soft round ends
- Reduce tissue ingrowth and hyperplasia reaction
- Visible green suture for easy removal
- Option of suture at both ends
- Radiopaque markers: 4 at both ends & 2 in middle
- Available with proximal or distal release and Through The Scope (TTS) options
- Available fully covered or with bare ends
Key Resources
Clinical Reference
- Outcomes Following Oesophageal Stent Insertion for Palliation of Malignant Strictures: A Large Single Center Series. Journal of Surgical Oncology 2012;105:60-65 Niall Lynch, et al.
- New Design Esophageal Stents for the Palliation of Dysphagia From Esophageal or Gastric Cardia Cancer: A Randomized Trial. Am J Gastroenterol 2008;103:304-312 Els M.L. Verschuur
- A new esophageal stent design (Niti-S stent) for the prevention of migration: a prospective study in 42 patients. GASTROINTESTINAL ENDOSCOPY Vol. 63, No.1: 2006 Peter D. Siersema